
There are bands (dark) where they are more likely to show up separated by bands (light) where they are less likely to show up. Particles going through two slits at once form an interference pattern on a screen in the far field. The signature of this is the so-called “interference pattern”: ripples in the distribution of where the particle is likely to be found at a screen in the far field beyond the slits, meaning a long way (often several metres) past the slits. Since we can’t know which slit the particle goes through, it acts as if it goes through both slits.

We also have a quantum particle with a position uncertainty large enough to cover both slits if it is fired at the barrier. In this type of experiment there is a barrier with two holes or slits.

We show a velocity disturbance - of the size expected from the Uncertainty Principle - always exists, in a certain sense.īut before getting into the details we need to explain briefly about the two-slit experiment. Heisenberg used the Uncertainty Principle to explain how measurement would destroy that classic feature of quantum mechanics, the two-slit interference pattern (more on this below).īut back in the 1990s, some eminent quantum physicists claimed to have proved it is possible to determine which of the two slits a particle goes through, without significantly disturbing its velocity.ĭoes that mean Heisenberg’s explanation must be wrong? In work just published in Science Advances, my experimental colleagues and I have shown that it would be unwise to jump to that conclusion. But this measurement would necessarily disturb its velocity, by an amount inversely proportional to the accuracy of the position measurement.
ACCORDING TO HEISENBERG UNCERTAINTY PRINCIPLE FULL
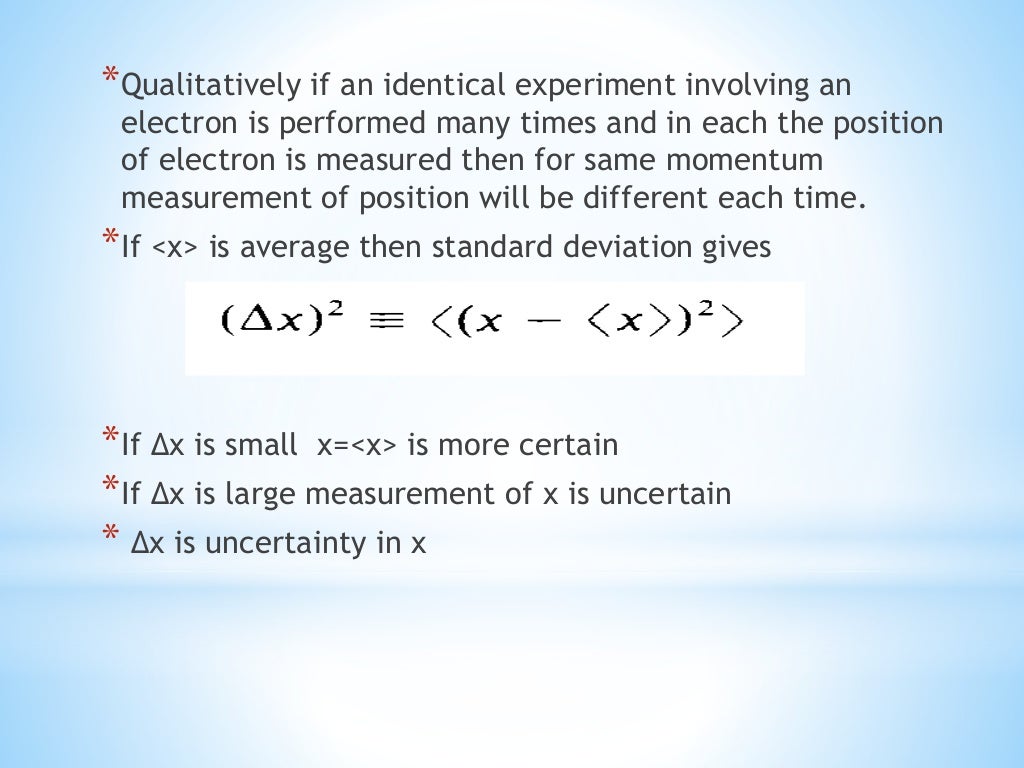
In the published 1927 paper, Heisenberg originally concluded that the uncertainty principle was Δ pΔ q ≈ h using the full Planck constant.For example, measuring the particle’s position would allow us to know its position. Introduced first in 1927 by the German physicist Werner Heisenberg, the uncertainty principle states that the more precisely the position of some particle is determined, the less precisely its momentum can be predicted from initial conditions, and vice versa. The uncertainty principle implies that it is in general not possible to predict the value of a quantity with arbitrary certainty, even if all initial conditions are specified. Such variable pairs are known as complementary variables or canonically conjugate variables and, depending on interpretation, the uncertainty principle limits to what extent such conjugate properties maintain their approximate meaning, as the mathematical framework of quantum physics does not support the notion of simultaneously well-defined conjugate properties expressed by a single value. In quantum mechanics, the uncertainty principle (also known as Heisenberg's uncertainty principle) is any of a variety of mathematical inequalities asserting a fundamental limit to the accuracy with which the values for certain pairs of physical quantities of a particle, such as position, x, and momentum, p, can be predicted from initial conditions. Uncertainty principle of Heisenberg, 1927. Canonical commutation rule for position q and momentum p variables of a particle, 1927.


 0 kommentar(er)
0 kommentar(er)
